
Natural lithium contains about 7.5 percent lithium-6.īoth natural isotopes have anomalously low nuclear binding energy per nucleon (compared to the neighboring elements on the periodic table, helium and beryllium) lithium is the only low numbered element that can produce net energy through nuclear fission.ħLi is one of the primordial elements (or, more properly, primordial nuclides) produced in Big Bang nucleosynthesis. Lithium-6 is valuable as the source material for the production of tritium (hydrogen-3) and as an absorber of neutrons in nuclear fusion reactions. Naturally occurring lithium is composed of two stable isotopes, 6Li and 7Li, the latter being the more abundant (92.5% natural abundance). Mass numbers of typical isotopes of Lithium are 6 7.

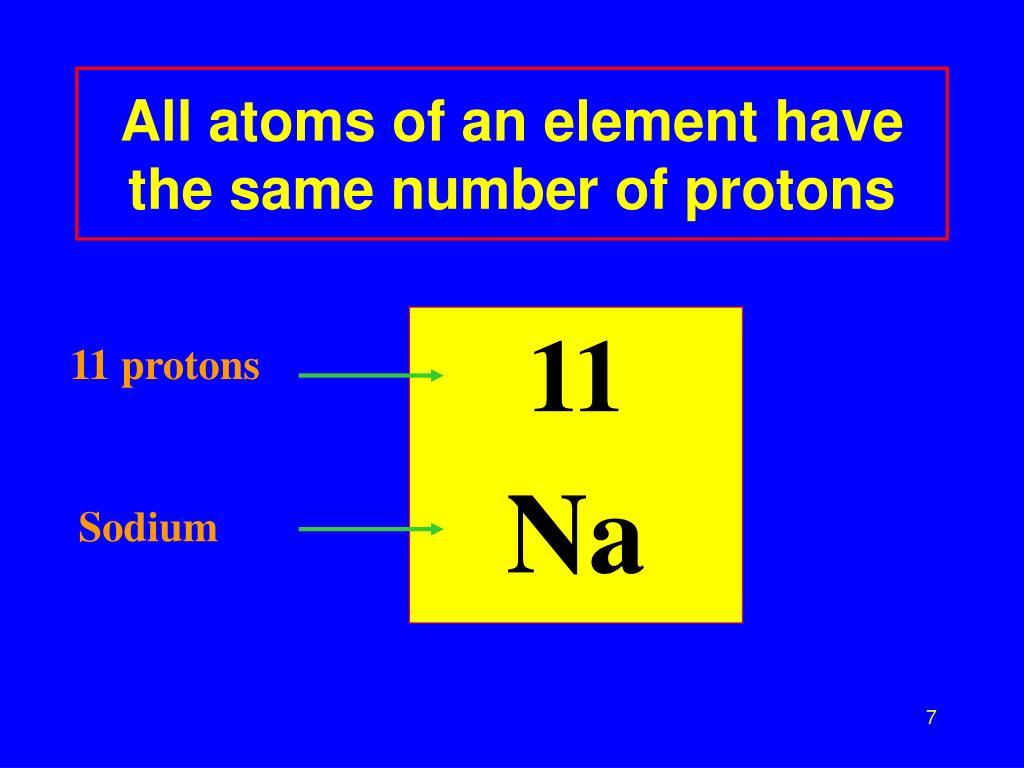
Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. The difference between the neutron number and the atomic number is known as the neutron excess: D = N – Z = A – 2Z.įor stable elements, there is usually a variety of stable isotopes. Neutron number plus atomic number equals atomic mass number: N+Z=A. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10 -19 coulombs. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. Lithium is a chemical element with atomic number 3 which means there are 3 protons in its nucleus.


 0 kommentar(er)
0 kommentar(er)
